How Do the Molecules of a Gas Behave
How Gases behave Week 4 Chemistry in Action. Each of the particles are well separated resulting in a very low density.
The atoms in a solid are so attracted to each other that they vibrate and dont move past each other.
. Gases need high temperatures and low pressures to behave ideally. Arrangement of Molecules in Solid Liquid and Gas. The behaviour of gas molecules is dependent on the properties and laws obeyed by the molecules of the gas.
All collisions both between the molecules themselves and between the molecules and the walls of the container are perfectly elastic. The distribution of molecules in a gas is very different from the distribution of molecules in liquids and solids. This chemical reaction released heat light and other products depending on the molecule being burnt.
Oxidation is the loss of electrons as the molecules break apart from each other and reform into different molecules. 2 H2 O2 2 H2O heat. Solid vibrate jiggle but generally do not move from place to place.
If at 120K the root-mean- square velocity of the gas molecules is V at 480K it becomes. These Molecules collide with one another and with anything else they come in contact With causing pressure. This is because the molecules of gases are faster at high temperatures and molecules have a large free volume around them at low pressures.
Gas molecules also move faster. When at a constant volume the addition of an inert gas causes no change in the equilibrium since the concentration of each species will remain constant I understand that there will be the. No gas can be perfectly ideal but real gases can behave like ideal ones.
Of course not all molecules move at the same speed and the range of speeds is described by the Boltzmann distribution assuming the gas molecules dont interact ie. I am well aware of Le Chateliers principle but what causes molecules to behave in such a way my guess is that it all has to do with rates of reactions and frequency of collisions. The move as far apart as possible in the container that holds them.
Particles are farther apart and move at high speed in all directions. The pressure of air pressing down on the Earth. A gas will fill any container but if the container is not sealed the gas will escape.
How do real gases behave. Pressure is due to collisions between the molecules and the walls of the container. Furthermore what is the particle arrangement of a liquid.
This can easily be observed by how fast odors appear in other rooms from where they originated in the homeoffice environment. Up to 24 cash back How Do Gas Molecules Behave. Gas can be compressed much more easily than a.
Carefully pull your bottles out of the bowl and make some ice. Put a thermometer in the ice and watch the temperature drop. They vibrate and move freely at high speeds.
Also know how do molecules behave in a liquid. How do the molecules of a gas behave. This is because gas particle have enough energy to overcome attractive forces.
Gas vibrate and move freely at high speeds. Gas molecules are free to move to fill the entire space of their container. This means that they diffuse rapidly throughout the container or volume in which they are contained.
Liquid vibrate move about and slide past each other. How do molecules behave in a gas state. Gas molecules are not held rigidly in place as would be a solid or a lattice of ionic bonds instead gas molecules are constantly in motion and each and every different gas exerts a specific pressure.
Hence we can say that air can behave like an ideal gas. How do the molecules of a gas behave quizlet. High temperature increases the kinetic energy of the gas molecules.
A substance with no definite shape or volume. When samples of gases are placed in. Answer choices As soon as the ice begins to melt.
The molecules of a liquid are attracted to each other but move more freely and past one another. How do atoms behave in a. Gases exert a push on things they are contained in.
Thank Writer Comment Blurt Anonymous answered Its either they vibrate in place or they clump together or they bounce around randomly Thank Writer. Gas expands the quickest because gas molecules are farther apart than molecules of other substances. Is air an ideal gas.
What are the properties of a Gas. For any given gas when the temperature is high and pressure is low that gas behaves like an ideal gas. If unconstrained gases will spread out indefinitely.
Molecules of gas move from an area of high concentration to an. If you put a thermometer into a pot of melting ice when will the temperature rise past zero. What element is a gas and can behave as an alkali metal.
Fill your bowl with ice rock salt and water bottles. If confined they will take the shape of their container. How do atoms behave in solid.
The average speed of air molecules ill be faster when heated and slower when cooled. Molecules of oxygen and other gases gas are in constant motion at room temperature Explanation. A gas will fill any container but if the container is not sealed the gas will escape.
The molecules behave as rigid spheres. There are five properties and five gas laws that govern the behaviour of gas molecules. An example is rocket fuel.
Solve any question of Kinetic Theory with-. Fill the bottom of a plate with water and put in the freezer. Gases are usually invisible and do not have a definite shape.
The atoms and molecules in gases are much more spread out than in solids or liquids. Answer choices They vibrate in place They bounce around randomly They are locked in a crystal lattice They clump together Question 10 120 seconds Q. Gases have no definite volume or shape.
Which is Hydrogen and Oxygen.

Deviation Of Gas From Ideal Behavior Boundless Chemistry

The Behavior Of Gases Chemistry For Non Majors
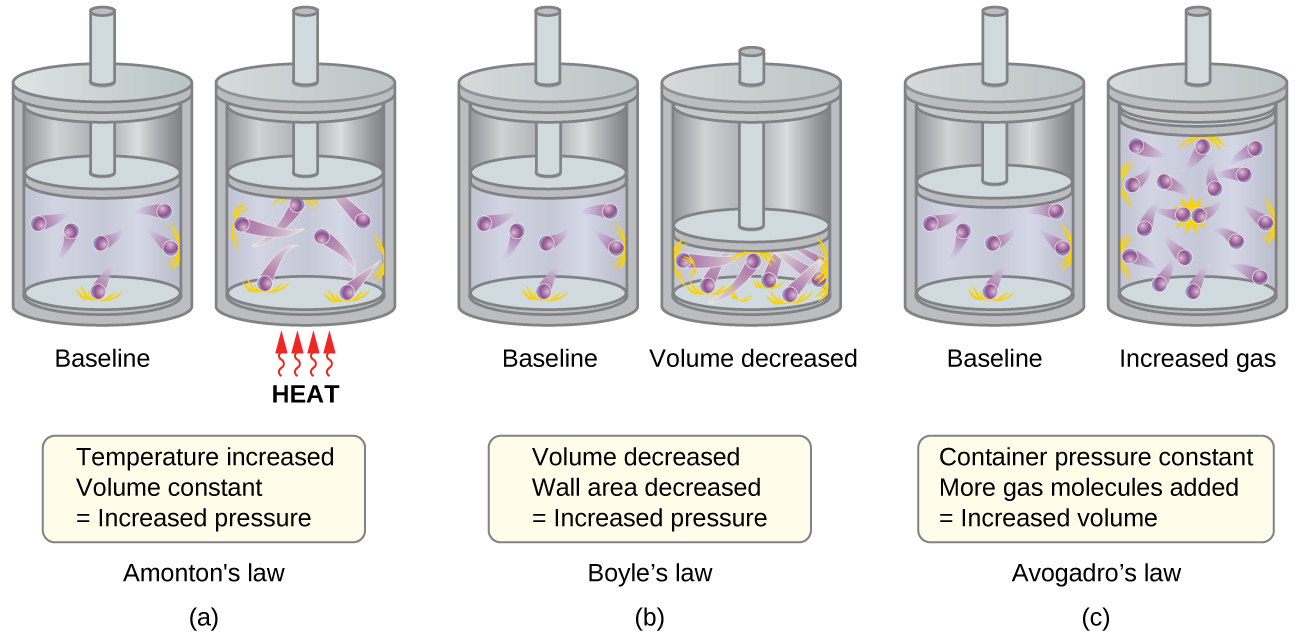
9 5 The Kinetic Molecular Theory Chemistry
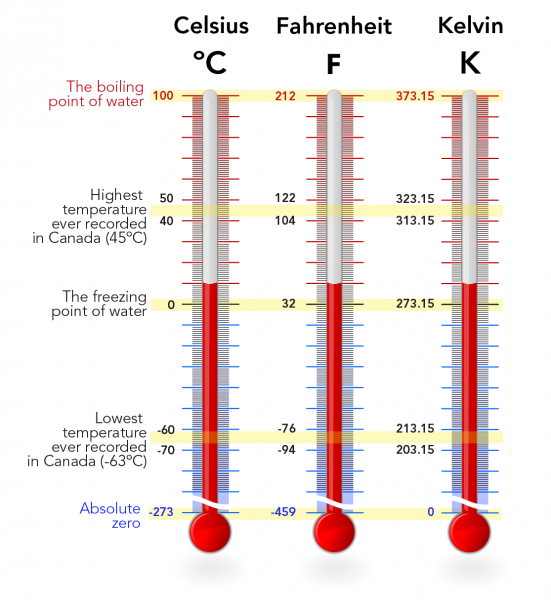
Kinetic Molecular Theory Of Gases Let S Talk Science
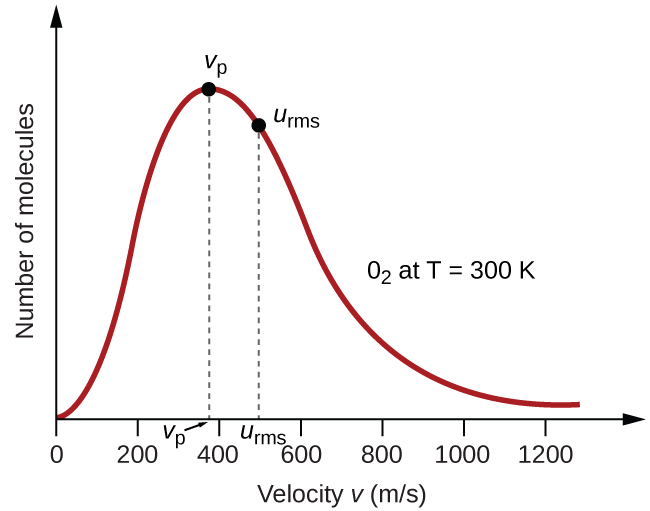
The Kinetic Molecular Theory Chemistry Atoms First 2e
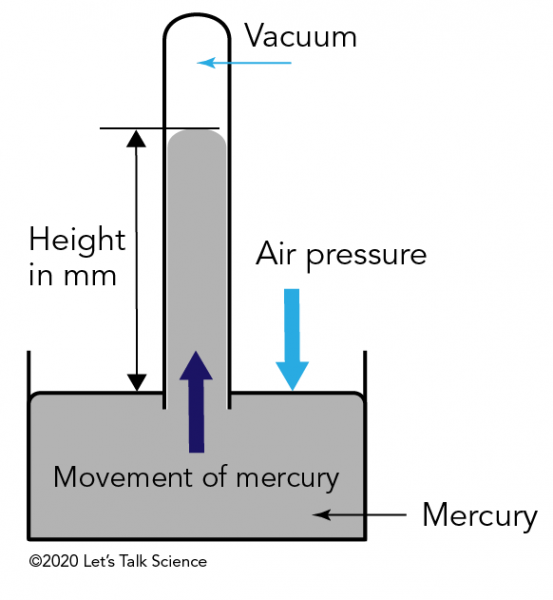
Kinetic Molecular Theory Of Gases Let S Talk Science
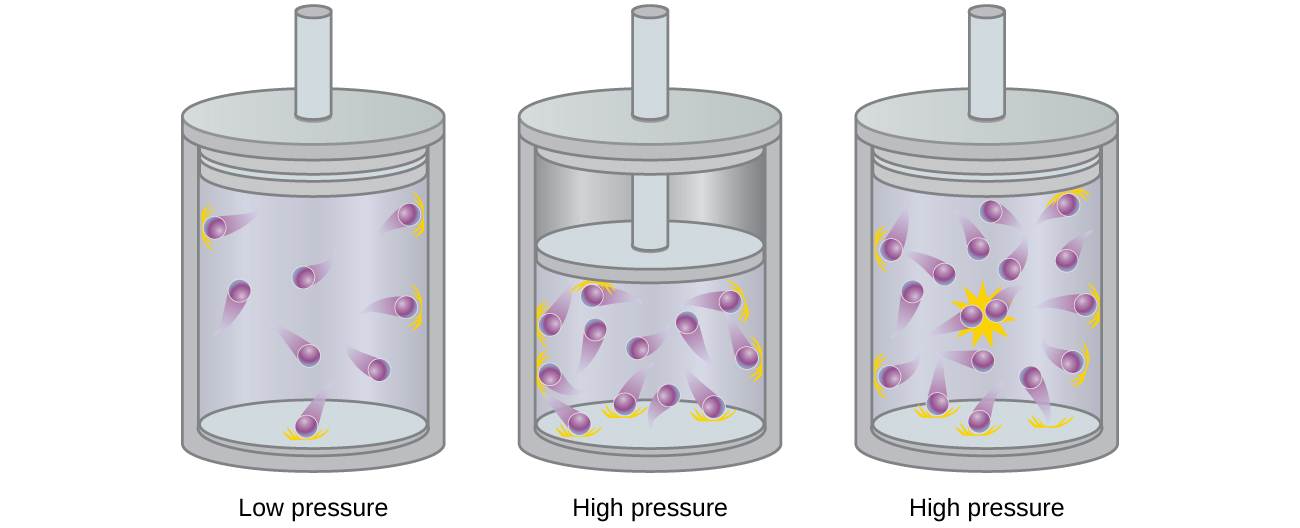
9 6 Non Ideal Gas Behavior Chemistry
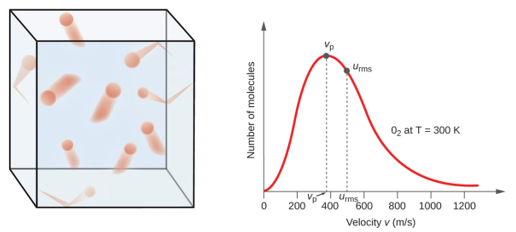
10 5 Kinetic Molecular Theory Of Gases Chemistry Libretexts
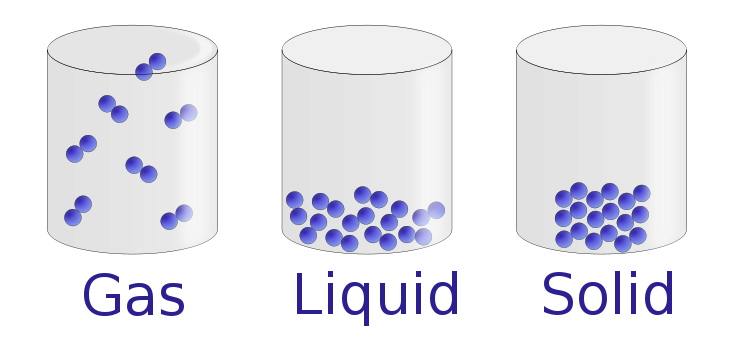
12 1 A Molecular Comparison Of Gases Liquids And Solids Chemistry Libretexts

10 5 Kinetic Molecular Theory Of Gases Chemistry Libretexts
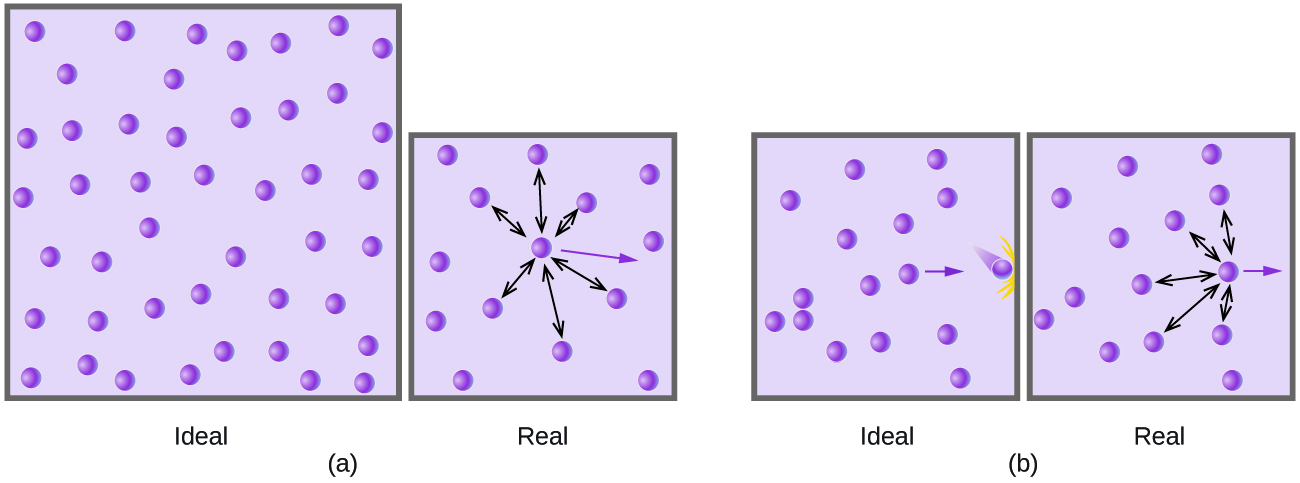
9 6 Non Ideal Gas Behavior Chemistry

Kinetic Molecular Theory Of Gases Practice Problems Youtube

The Kinetic Molecular Theory Of Gases Ck 12 Foundation
Kinetic Molecular Theory Of Gases Introductory Chemistry 1st Canadian Edition

Comments
Post a Comment